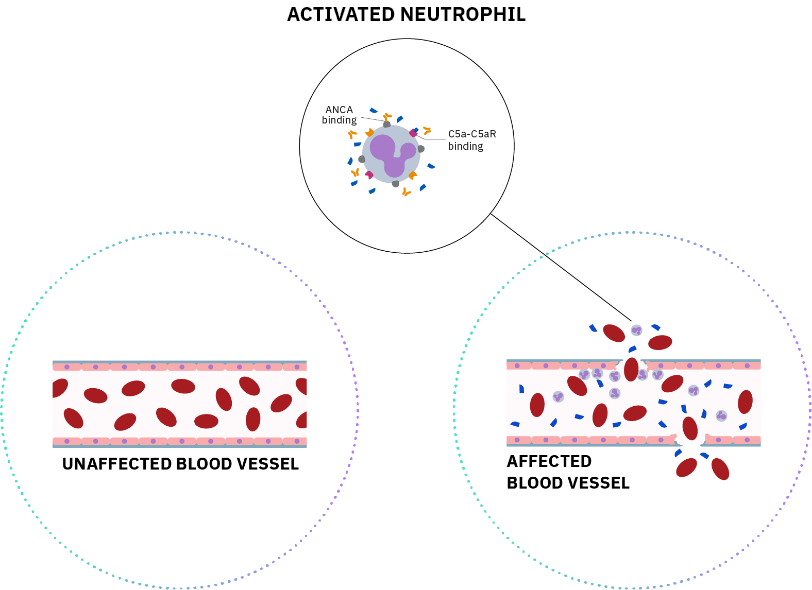
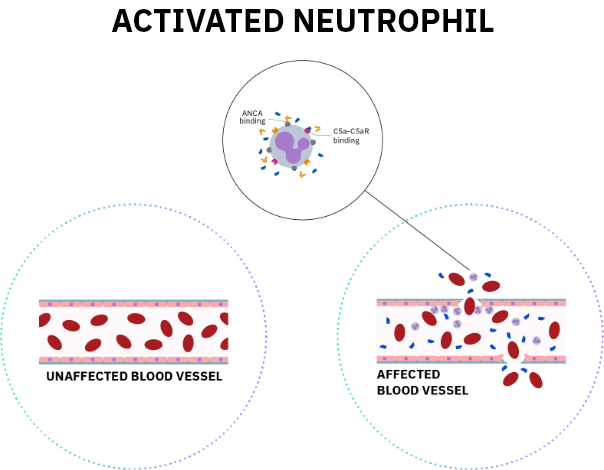
References:
1. Sed ut
perspiciatis unde omnis iste natus error sit voluptatem accusantium doloremque
laudantium, totam rem aperiam, eaque ipsa quae ab illo inventore veritatis et quasi
architecto beatae vitae dicta sunt explicabo. Nemo enim ipsam voluptatem quia
voluptas sit aspernatur aut odit aut fugit, sed quia consequuntur magni dolores eos
qui ratione voluptatem sequi nesciunt. Neque porro quisquam est, qui dolorem ipsum
quia dolor.
References: 1. TAVNEOS [package insert]. Cincinnati, OH: Amgen Inc.
2. Data on file, Amgen; Clinical Study Report [92070]; 2020.
3. Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group. N Engl J Med. 2021;384(7):599-609.
4. Terrier B, Pagnoux C, Perrodeau E, et al; French Vasculitis Study Group. Ann Rheum Dis. 2018;77(8):1151-1157.
5. Data on file, Amgen; Study Supplied [91955]; 2021.
6. Chung SA, Langford CA, Maz M, et al. Arthritis Rheumatol. 2021;73(8):1366-1383.
7. Mukhtyar C, Lee R, Brown D, et al. Ann Rheum Dis. 2009;68(12):1827-1832.
References: 1. Chung SA, Langford CA,
Maz M, et al. Arthritis Rheumatol.
2021;73(8):1366-1383. 2. Neumann I.
Rheumatology (Oxford). 2020;59(Suppl
3):iii60-iii67. 3. Data on file, Amgen. Clinical Study Report
[92070];2020. 4. King C, Druce KL,
Nightingale P, et al. Rheumatol Adv Pract. 2021;5(3):rkab018. 5.
Jain K, Jawa P, Derebail VK, et al. Kidney360.
2021;2(4):763-770. 6. Kitching AR, Anders H-J, Basu N, et al.
Nat Rev Dis Primers. 2020;6(1):71. 7. Yates M, Watts R.
Clin Med (Lond). 2017;17(1):60-64. 8.
Robson J, Doll H, Suppiah R, et al. Rheumatology (Oxford). 2015;54(3):471-481.
9. Geetha D, Jefferson JA. Am J Kidney Dis.
2020;75(1):124-137. 10. Supplement to: Walsh M, Merkel PA, Peh
C-A, et al; PEXIVAS Investigators. N Engl J Med. 2020;382(7):622-631.
11. Terrier B, Pagnoux C, Perrodeau E, et al.
Ann Rheum Dis. 2018;77(8):1151-1157. 12.
Guillevin L, Pagnoux C, Karras A, et al. N Engl J Med. 2014;371(19):1771-1780.
13. Data on file, Amgen. Harris Poll [91973]; 2022.
14. Robson JC, Dawson J, Cronholm PF, et al. Patient Relat
Outcome Meas. 2018;9:17-34. 15. Jayne
DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group. N Engl J Med. 2021;384(7):599-609.
References: 1. TAVNEOS [package insert]. Cincinnati, OH: Amgen Inc.
2. Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study
Group. N Engl J Med. 2021;384(7):599-609.
3. Data on
file, Amgen. Clinical Study Report [92070]; 2020. 4.
Supplement to: Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group. N Engl J Med.
2021;384(7):599-609. 5. Data on file, Amgen. [92252]; 2020.
6. Kaplan-Pavlovčič S, Cerk K, Kveder R, et al. Nephrol Dial
Transplant. 2003;18(suppl 5):v5-v7.
7. Stangou M, Asimaki A, Bamichas G, et al. J Nephrol.
2005;18(1):35-44. 8. Data on file, Amgen. [92757]; 2020.
9. Preedy VR, Watson RR, eds.
Handbook of Disease Burdens and Quality of Life Measures. Springer Science+Business Media; 2010.
10. Data on file, Amgen; Study Supplied [91955]; 2021.
11. Hellmich B, Sanchez-Alamo B, Schirmer JH, et al.
Ann Rheum Dis. 2023. doi: 10.1136/ard-2022-223764
References: 1. Samman KN, Ross C, Pagnoux C, Makhzoum J-P. Int J Rheumatol. 2021;2021:5534851.
2. TAVNEOS [package insert]. Cincinnati, OH: Amgen Inc.
3. Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study
Group. N Engl J Med. 2021;384(7):599-609.
References: 1. TAVNEOS [package insert]. Cincinnati, OH: Amgen Inc.
2. Chung SA,
Langford CA, Maz M, et al. Arthritis Rheumatol. 2021;73(8):1366-1383.
References: 1. TAVNEOS [package
insert]. Cincinnati, OH: Amgen Inc. 2. Chung SA, Langford CA, Maz
M, et al. Arthritis Rheumatol. 2021;73(8):1366-1383.
References: 1. TAVNEOS [package
insert]. Cincinnati, OH: Amgen Inc. 2. Chung SA, Langford CA, Maz
M, et al. Arthritis Rheumatol. 2021;73(8):1366-1383.
References: 1. TAVNEOS [package insert]. Cincinnati, OH: Amgen Inc.
2. Data on file, Amgen; Clinical Study Report [92070]; 2020.
3. Khan MM, Molony DA. Ann Intern Med. 2021;174(7):JC79.
4. Chen M, Jayne DRW, Zhao M-H. Nat Rev Nephrol. 2017;13(6):359-367.
5. Kitching AR, Anders H-J, Basu N, et al. Nat Rev Dis Primers. 2020;6(1):71.
6. Al-Hussain T, Hussein MH, Conca W, Al Mana H, Akhtar M. Adv Anat Pathol. 2017;24(4):226-234.
7. Jennette JC, Nachman PH. Clin J Am Soc Nephrol. 2017;12(10):1680-1691.
8. Shochet L, Holdsworth S, Kitching AR. Front Immunol. 2020;11:525.
9. Jones RB, Ferraro AJ, Chaudhry AN, et al. Arthritis Rheum. 2009;60(7):2156-2168.
10. Winkelstein A. Blood. 1973;41(2):273-284.
11. Eickenberg S, Mickholz E, Jung E, et al. Arthritis Res Ther. 2012;14(3):R110.
12. Ogino MH, Prasanna T. In: StatPearls [Internet]. StatPearls Publishing; 2023. Accessed October 3, 2023. https://www.ncbi.nlm.nih.gov/books/NBK553087/
13. Mohammadi O, Kassim TA. In: StatPearls [Internet]. StatPearls Publishing; 2023.
14. Anders H-J, Nakazawa D. CJASN. 2021;16:1581-1583.
References: 1. Smith RM, Jones RB,
Jayne DRW. Arthritis Res
Ther. 2012;14(2):210. 2. Berti A, Dejaco C. Best
Pract Res Clin Rheumatol.
2018;32(2):271-294. 3. Berden A, Göçeroğlu A, Jayne D, et al.
BMJ. 2012;344:e26. 4. Jennette
JC, Falk RJ, Bacon PA, et al. Arthritis Rheum. 2013;65(1):1-11.
5. Mukhtyar CB. General presentation of the vasculitides. In:
Watts RA, Scott DGI, eds. Vasculitis in Clinical
Practice. Springer; 2010:13-19. 6.
Terrier B, Darbon R, Durel C-A, et al. Orphanet J Rare Dis.
2020;15(suppl2):351. 7. Chung SA, Langford CA, Maz M, et al.
Arthritis Rheumatol. 2021;73(8):1366-1383.
8. Lamprecht P, Kerstein A, Klapa S, et al. Front
Immunol. 2018;9:680. 9.
Al-Hussain T, Hussein MH, Conca W, et al. Adv Anat Pathol. 2017;24(4):226-234.
10. Qasim A, et al. In: StatPearls [Internet]. StatPearls
Publishing; 2023. Accessed March 31, 2023. https://www.ncbi.nlm.nih.gov/books/NBK554372/
11. Hunter
RW, Welsh N, Farrah TE, et al.
BMJ. 2020;369:m1070. 12. Chen M, Jayne DRW, Zhao M-H.
Nat Rev Nephrol. 2017;13(6):359-367. 13. Yates M, Watts
R. Clin Med (Lond).2017;17(1):60-64.
14. Syed R, Rehman A, Valecha G, et al. Biomed Res Int.
2015;2015:402826.
References: 1. Lamprecht P,
Kerstein A, Klapa S, et al. Front Immunol. 2018;9:680.
2. Chung SA, Langford CA, Maz M, et al. Arthritis
Rheumatol. 2021;73(8):1366-1383. 3. Data on file, Amgen.
Clinical Study Report [92070];2020. 4. Jayne DRW, Merkel
PA, Schall TJ, Bekker P; ADVOCATE Study Group. N Engl J Med.
2021;384(7):599-609. 5. Supplement to: Jayne DRW, Merkel PA,
Schall TJ, Bekker P; ADVOCATE Study Group. N Engl J Med. 2021;384(7):599-609.
6. Mukhtyar CB. General presentation of the vasculitides. In:
Watts RA, Scott DGI, eds. Vasculitis in Clinical
Practice. Springer; 2010:13-19. 7. Geetha D, Jefferson
JA. Am J Kidney Dis.
2020;75(1):124-137. 8. Salama AD. Kidney Int Rep.
2020;5(1):7-12. 9.
Hellmich B, Sanchez-Alamo B, Schirmer JH, et al. Ann Rheum Dis. 2023. doi:
10.1136/ard-2022-223764
10. Kitching AR, Anders H-J, Basu N, et al. Nat Rev Dis
Primers. 2020;6(1):71.
11. Jariwala MP, Laxer RM. Front Pediatr. 2018;6:226.
12. Macarie SS,
Kadar A. Rom J Ophthalmol. 2020;64(1):3-7. 13. Palmucci
S, Inì C, Cosentino S, et al.
Diagnostics (Basel). 2021;11(12):2318. 14.
Lionaki S, Skalioti C, Marinaki S, et al. Pauci-immune vasculitides with kidney
involvement. In: Mohammed RHA, ed.
Vasculitis in Practice: An Update on Special Situations – Clinical and Therapeutic
Considerations. InTechOpen; 2018.
15. Yang J, Li M. BMJ. 2022;376:e065658.
16.
Kermani TA, Cuthbertson D,
Carette S, et al; Vasculitis Clinical Research Consortium. J Rheumatol.
2016;43(6):1078-1084.
17. Merkel PA, Aydin SZ, Boers M, et al. J Rheumatol.
2011;38(7):1480-1486.
18. Mukhtyar C, Lee R, Brown D, et al. Ann Rheum Dis.
2009;68(12):1827-1832. 19. Pagnoux C. Eur J Rheumatol.
2016;3(3):122-133.
20. Data on file, Amgen. LabCorp Sample [93232]; 2022.
21. Zagelbaum N, Shamim Z,
Gilani A, et al. Pulm Crit Care Med. 2016;1(3):1-4.
References: 1. Data on file,
Amgen. TAVNEOS Payer Approval Percentage [93621]; 2023.
2. Data on file, Amgen. TAVNEOS Time to First Drug Shipment
[93622]; 2023. 3. Data on file, Amgen. Patient and Prescriber
Counts [93239]; 2023.
References:
1. Sed ut
perspiciatis unde omnis iste natus error sit voluptatem accusantium doloremque
laudantium, totam rem aperiam, eaque ipsa quae ab illo inventore veritatis et quasi
architecto beatae vitae dicta sunt explicabo. Nemo enim ipsam voluptatem quia
voluptas sit aspernatur aut odit aut fugit, sed quia consequuntur magni dolores eos
qui ratione voluptatem sequi nesciunt. Neque porro quisquam est, qui dolorem ipsum
quia dolor.